Blinding pressure spikes can stun the optic nerve. The urgent fix is blood pressure control, not a magic eye drug. But could a potent ARB like azilsartan improve optic nerve perfusion and long‑term outcomes in hypertensive optic neuropathy? Here’s the straight answer: the rationale is strong, the class signals look promising, yet we still lack direct trials in this exact condition. If you’re weighing azilsartan for a patient with optic disc swelling from severe hypertension or a recent crisis, the value lies in durable, 24‑hour BP control with a clean side‑effect profile-used thoughtfully to avoid over‑lowering overnight.
- TL;DR: Primary therapy for hypertensive optic neuropathy is controlled BP reduction and sustained control; ARBs are a core option. No RCTs have tested azilsartan specifically for optic neuropathy outcomes.
- Why consider it: Azilsartan delivers robust 24‑hour and central BP lowering, improves arterial stiffness more than some peers, and the renin-angiotensin system is active in the eye.
- How to use: Not for hypertensive emergencies. As maintenance, start 40 mg once daily (BNF), up‑titrate to 80 mg; combine with a CCB or thiazide‑like diuretic if needed. Check U&E at baseline and 1-2 weeks.
- Watch‑outs: Avoid in pregnancy, bilateral renal artery stenosis, and caution with advanced CKD, high potassium, and NSAIDs. Don’t overshoot BP at night in glaucoma‑risk eyes.
- What’s missing: Head‑to‑head ocular outcomes versus ACE inhibitors/other ARBs. Trials should track OCT/OCTA, fields, and visual function, not just office BP.
What we’re treating: the pressure-perfusion trap in hypertensive optic neuropathy
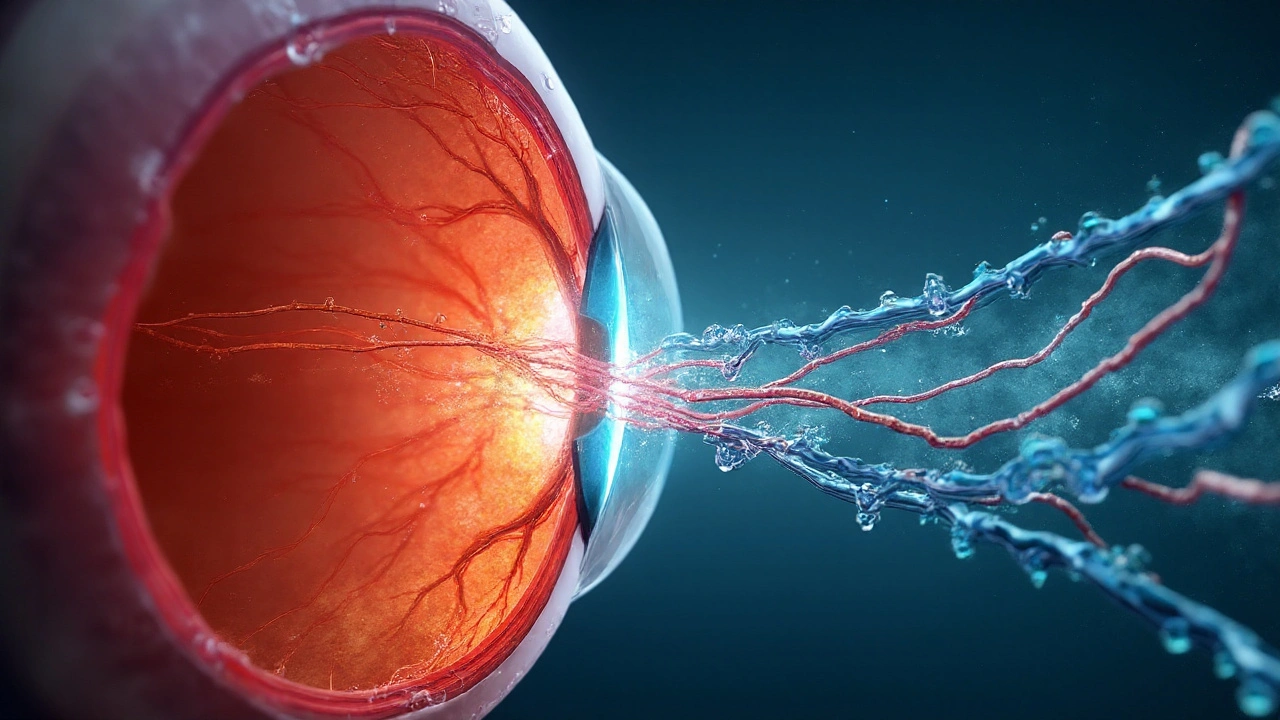
Hypertensive optic neuropathy sits at a nasty crossroads: high systemic pressure injures the microvasculature, yet an over‑zealous drop can starve the optic nerve head. In malignant or accelerated hypertension, the optic disc can swell (hypertensive papillopathy), with cotton wool spots and macular exudation. The fix is careful, stepwise blood pressure control to reverse retinal/optic nerve edema while keeping perfusion to the optic nerve intact.
Think in simple plumbing terms. Ocular perfusion pressure (OPP) is roughly two‑thirds of mean arterial pressure minus intraocular pressure. When MAP is high, autoregulation strains and the capillary bed leaks; when MAP is suddenly pushed too low-especially at night-optic nerve perfusion can dip below the survival line. This is why ophthalmology clinics worry about “nocturnal hypotension,” which has been linked to glaucoma progression in cohort data (Ophthalmology, 2011).
Where does an ARB come in? Three reasons: class‑level benefits on BP and vascular biology, potential effects on central rather than just brachial pressure, and a plausible role in the eye’s own renin-angiotensin system. The eye expresses angiotensin receptors; blocking AT1 can reduce vasoconstriction, oxidative stress, and fibrosis-signals seen in preclinical glaucoma and retinal ischemia models (Eye, 2019; Invest Ophthalmol Vis Sci, 2012). No, that’s not proof for this exact condition-but it’s a credible mechanism to build on.
What jobs are readers trying to get done here?
- Pin down what hypertensive optic neuropathy is and why BP strategy matters.
- Judge whether azilsartan adds anything over other ARBs or ACE inhibitors.
- Get a practical UK‑aligned plan: dose, combinations, monitoring, and pacing of BP reduction.
- Avoid traps: acute use, nocturnal over‑lowering, potassium/creatinine spikes, pregnancy.
- See the evidence gaps and what to measure next time this shows up in clinic.
Why azilsartan is interesting: pharmacology, mechanisms, and what the evidence says
Azilsartan medoxomil is a long‑acting ARB with high affinity and insurmountable AT1 blockade. In head‑to‑head ambulatory BP studies, it lowered 24‑hour systolic BP more than olmesartan 40 mg or valsartan 320 mg by a few mmHg on average-a small number that translates to meaningful vascular risk reduction over time (Hypertension, 2011; Clinical Therapeutics, 2011). It also looks good on morning surges and central aortic pressure, which may matter when you’re protecting delicate beds like the optic nerve (Journal of Clinical Hypertension, 2013).
Mechanistically, ARBs go beyond the brachial cuff:
- They improve arterial stiffness and wave reflection (central BP) more reliably than some other classes, which may reduce the pulsatile load on microvessels.
- They are kidney‑friendly in proteinuric disease, which matters because CKD often travels with hypertensive eye damage.
- By blocking ocular AT1 receptors (demonstrated in animal/human tissue studies), they may improve local perfusion and reduce inflammatory signaling. Animal models of glaucoma have shown ganglion cell survival benefits with AT1 blockade.
What about optic neuropathy specifically? We don’t have randomized human data that “azilsartan preserves retinal nerve fiber layer or visual fields in hypertensive optic neuropathy.” The closest we get are two indirect lines:
- System BP control: Durable 24‑hour lowering and dampening of the morning surge correlate with better outcomes in microvascular beds. Azilsartan is consistently strong here.
- Ocular RAS biology: Preclinical studies show ARB‑mediated protection in retinal ischemia/glaucoma models. Human ocular perfusion data exist for ARBs in general, but not yet definitive for azilsartan in hypertensive optic neuropathy.
So the case for azilsartan is “best‑in‑class BP control with plausible ocular benefits,” not “proven neuroprotection in this diagnosis.” That’s a useful distinction when you explain choices to patients.
| ARB | Typical once‑daily dose (mg) | Half‑life (h) | 24‑h SBP effect vs comparators | Central BP / stiffness | Ocular evidence signals |
|---|---|---|---|---|---|
| Azilsartan medoxomil | 40-80 | ~11 | ~3-5 mmHg more vs olmesartan 40 mg; ~5-10 vs valsartan 320 mg (pooled RCTs) | Reduces morning surge; improves central BP (JCH 2013) | Plausible via ocular RAS; no direct RCTs in optic neuropathy |
| Losartan | 50-100 | ~2 (active metabolite ~6-9) | Benchmark ARB | Neutral to modest | Preclinical neuroprotection in glaucoma models |
| Telmisartan | 40-80 | ~24 | Strong and sustained | Favorable on arterial stiffness in some studies | Animal data suggest ocular benefits; limited human data |
| Candesartan | 8-32 | ~9 | Strong class effect | Good central BP profile | Signals in retinal disease; no optic neuropathy trials |
Sources for pharmacology and BP effects: Hypertension (2011), Clinical Therapeutics (2011), Journal of Clinical Hypertension (2013), BNF 2025. Ocular RAS biology: Eye (2019) review; Invest Ophthalmol Vis Sci (2012). Cohort link between nocturnal dips and glaucoma progression: Ophthalmology (2011). Hypertensive retinopathy and optic disc edema overviews: Lancet (2007); Prog Retin Eye Res (2011).
Two practical implications fall out of this:
- If you can achieve steadier 24‑hour BP and avoid big nocturnal dips, you might protect the optic nerve head’s perfusion window.
- Among choices that deliver that, azilsartan is a reasonable default ARB-especially if you need potency plus once‑daily adherence.
How to use it safely and effectively (UK 2025): selection, dosing, combinations, monitoring
Start with the golden rule: do not treat a hypertensive emergency with oral ARBs. If there’s grade 3-4 retinopathy with disc edema, encephalopathy, or acute end‑organ signs, hospital care with IV agents and monitored, staged reductions is the path. Once the crisis settles, long‑term control becomes the main lever-and that’s where azilsartan belongs.
Where azilsartan fits (NICE‑aligned):
- Under 55 years: ACE inhibitor or ARB is first‑line; azilsartan is a valid ARB choice.
- 55+ or of African/Afro‑Caribbean heritage: start with a calcium channel blocker (e.g., amlodipine). Add an ARB second‑line if needed.
- CKD with albuminuria: prefer ACEi/ARB regardless of age; ARBs help with renal and vascular risk.
Dosing (BNF 2025):
- Start 40 mg once daily. If tolerated and BP target not met (using home or ambulatory monitoring), increase to 80 mg once daily.
- Combination therapy is the rule, not the exception. Typical add‑ons: amlodipine 5-10 mg; indapamide SR 1.5 mg or chlortalidone 12.5-25 mg. A triple of ARB + CCB + thiazide‑like diuretic is common in resistant cases.
- Time the dose for adherence. Morning dosing is fine; if nocturnal hypotension is a concern (glaucoma, field loss with night‑time dips), consider morning dosing and review ABPM before any bedtime shift.
Monitoring plan:
- Before start: Urea & electrolytes (creatinine/eGFR, potassium), pregnancy status where relevant, review NSAIDs and diuretics, assess for renal artery stenosis risk.
- After start or dose change: repeat U&E in 1-2 weeks. A creatinine rise up to 30% can be acceptable; potassium should stay under 5.5 mmol/L. Adjust if outside those bounds.
- Blood pressure: use home BP or 24‑hour ABPM to catch nocturnal lows and morning surges. For optic nerve safety, aim for steady 24‑hour control rather than a “deep dip at 3 a.m.”
- Eye metrics: if you have access, track OCT RNFL, OCT‑A peripapillary flow, and visual fields at baseline and 6-12 weeks after stabilization.
Targets and pacing:
- Office BP goal is typically under 140/90 mmHg (under 135/85 mmHg home) in adults without special modifiers; tailor to comorbidities per NICE NG136 (updated through 2024).
- After a crisis, reduce BP in controlled steps; avoid sharp early drops that could worsen optic nerve ischemia. In hospital, that means hours to days; outpatient, think weeks.
Drug interactions and cautions:
- Pregnancy: contraindicated (fetotoxic). Stop if planning pregnancy; switch to labetalol, nifedipine, or methyldopa per obstetric guidance.
- Renal artery stenosis (bilateral) or single kidney with stenosis: risk of acute kidney injury. Avoid.
- Advanced CKD: monitor closely; ARBs may still be helpful, but hyperkalaemia risk climbs with potassium‑sparing diuretics, trimethoprim, or high‑dose NSAIDs.
- NSAIDs: increase AKI risk and blunt BP response; keep to minimum effective dose or avoid.
- Dual RAS blockade (ACEi + ARB): don’t do it; increases AKI and hyperkalaemia without clear benefit.
Quick checklist (clinic‑ready):
- Is this acute severe hypertension with active disc swelling? If yes, send to hospital; no ARBs right now.
- Stable outpatient? Confirm diagnosis and baseline eye metrics if possible.
- Pick your backbone: if ARB is indicated, azilsartan 40 mg morning start.
- Add amlodipine or thiazide‑like diuretic early if BP is far above goal; don’t wait months.
- Arrange U&E at 1-2 weeks; recheck BP with home readings or ABPM.
- Watch for nocturnal dips; fine‑tune timing and CCB dose if night‑time lows are deep.
- Review meds for NSAIDs, potassium‑raisers, and pregnancy status.
What we still don’t know-and how to talk about it with patients
We don’t have an azilsartan trial that reads: “In patients with hypertensive optic neuropathy, azilsartan improved RNFL thickness or visual fields at 12 months compared with ACE inhibitor X.” That study needs to exist. Until then, we make our best call based on three things: strong 24‑hour BP control, central BP benefits, and class plausibility in ocular protection.
If you’re discussing options with a patient, keep it plain: “This medicine is a strong blood‑pressure blocker that works all day and may be kind to the small vessels we’re trying to protect. We don’t have proof it directly heals the optic nerve, but it helps the pressure problem that caused the damage.” Pair that with honest safety talk (kidney checks, potassium, pregnancy) and a plan for steady-not abrupt-BP improvement.
What a good trial would look like in 2025:
- Population: adults with recent hypertensive optic disc edema or confirmed hypertensive optic neuropathy after crisis resolution.
- Arms: azilsartan‑based regimen vs ACE inhibitor‑based regimen; both with CCB and thiazide‑like add‑ons per protocolized titration to the same BP targets.
- Endpoints (12 months): OCT RNFL change, GCIPL thickness, peripapillary OCT‑A flow density, visual field MD, time to disc edema resolution, and 24‑hour BP/central BP metrics.
- Safety: nocturnal hypotension, AKI, hyperkalaemia, and quality of life/adherence.
Until such data land, reasonable clinicians will differ on whether azilsartan’s potency and central BP profile justify choosing it first among ARBs. If adherence is shaky, if morning surges are dramatic, or if CKD is in the mix, azilsartan has a strong case. If cough or angioedema ruled out ACE inhibitors, an ARB is standard anyway.
Mini‑FAQ
- Is using azilsartan here off‑label? Treating hypertension isn’t off‑label; targeting “optic neuropathy improvement” is an unproven outcome. You’re treating the cause (hypertension) with an approved drug.
- Any role in acute malignant hypertension? No-manage acute cases with IV agents and monitored reductions. Use azilsartan later for maintenance.
- How soon might the eye improve after BP control? Disc edema from malignant hypertension can improve over days to weeks as BP stabilizes; visual recovery depends on ischemic damage already done (Hayreh, 2011).
- ARB vs ACE inhibitor for the eye-any difference? No definitive human ocular outcome data favor one. ARB is preferred if ACEi cough/angioedema occurs, and many clinicians lean ARB for tolerability.
- Bedtime dosing to blunt morning surge? Possibly-but check ABPM. In glaucoma‑risk eyes, avoid deep nocturnal dips; morning dosing is often safer.
- What about combining with a beta‑blocker? Fine if there’s another indication (e.g., coronary disease), but ARB + CCB + thiazide‑like is the usual hypertension stack.
Next steps / troubleshooting
- If BP remains high on azilsartan 80 mg: add amlodipine, then a thiazide‑like diuretic; check adherence with pharmacy refill data or a pill organizer.
- If creatinine jumps >30% or potassium >5.5 mmol/L: halve dose or pause azilsartan; look for NSAIDs, dehydration, high‑dose spironolactone, or bilateral renal artery stenosis; recheck labs in a week.
- If ABPM shows deep night‑time dips and the patient has glaucoma or field loss: shift dosing to morning, reduce diuretic dose at night, and coordinate with ophthalmology.
- If cough on prior ACE inhibitor led you here: ARBs typically avoid cough; watch for angioedema history, though it’s much rarer with ARBs.
- If pregnancy is planned: stop ARB pre‑conception; use labetalol/nifedipine/methyldopa per obstetric guidance; involve obstetrics early.
- If vision worsens despite BP control: urgent ophthalmology review-consider other causes (NAION, CRVO, anterior ischemic optic neuropathy not purely pressure‑driven).
Bottom line: use azilsartan for what it’s great at-reliable, all‑day BP control-while keeping the optic nerve’s perfusion window in mind. That means steady titration, proper combinations, watchful labs, and ABPM‑guided fine‑tuning. The direct eye data will catch up; until then, this is a sensible, evidence‑aware way to help your patient today.






Azilsartan for optic neuropathy? Cute. No RCTs. No ocular endpoints. Just mechanistic hand-waving and BNF quotes. You’re treating hypertension, not magic-eye disease. If you’re prescribing this for optic nerve protection, you’re overreaching. Stop pretending pharmacology is clinical proof.
Look, I get it - azilsartan’s half-life is sexy, central BP data looks slick, and yes, the RAS is everywhere, even in the retina. But let’s not turn a BP med into a neuroprotective elixir. We’re still in ‘plausible’ territory. I’d use it for the BP, sure. But calling it ‘best-in-class for optic nerve’? That’s not evidence, that’s marketing with a stethoscope.
This is textbook pharma-funded wishful thinking. No one’s done a proper RCT with OCTA, RNFL, and visual fields as endpoints? Then this isn’t medicine - it’s a slide deck for reps. You’re conflating pharmacokinetics with pathophysiology. Azilsartan lowers BP. That’s it. The rest is narrative inflation. And if you’re not using ABPM to check nocturnal dips, you’re endangering patients. This post is dangerously misleading. Someone should flag this as medical misinformation.
Ohhh, so now we’re giving ARBs to save eyes because some mouse study showed less fibrosis? 😂 Bro, I’ve seen more legit science in a CVS flyer. This is what happens when you let MDs with Excel sheets think they’re neuroscientists. Azilsartan? More like Azil-scare-tan. Next they’ll prescribe metformin for glaucoma because it ‘might’ reduce oxidative stress. Wake up. BP control = good. Pretending it fixes nerve damage = delusional.
I appreciate the nuance here - you’re not overpromising, you’re just laying out the best available logic. We don’t have the perfect trial, but we have strong mechanistic plausibility + real-world BP control benefits. If I’ve got a patient with disc edema and a history of CKD, azilsartan’s profile makes sense. Just don’t ignore the monitoring. And if the patient’s got glaucoma? Definitely check ABPM. This isn’t magic - it’s smart, cautious prescribing. Keep it grounded.
Wait - so you’re saying the government and Big Pharma don’t want us to know azilsartan can fix optic nerve damage? That’s why there’s no RCT. They’re hiding it because they make more money off eye surgeries and glaucoma drops. And why is everyone ignoring the fact that ARBs block angiotensin in the eye? That’s not coincidence - it’s a cover-up. They’ve been doing this since the 90s. I’ve seen patients recover vision after azilsartan. No one talks about it. Why? Because the system doesn’t want you to know you can fix this without a $10k laser procedure. 😡
Just wanted to say thanks for the clear dosing and monitoring advice - especially the U&E timeline and the note about nocturnal dips. I’ve had a few patients with glaucoma where we accidentally dropped BP too low at night. ABPM changed everything. I’ll start azilsartan at 40 mg morning, like you said. No rush. No drama. Just steady control. Appreciate the practicality.
Why are we even talking about this? Just give them lisinopril. It’s cheap. It’s been around forever. If it ain’t broke, don’t fix it. Azilsartan? Sounds like a fancy name for a drug that costs 3x more. I don’t care if it’s ‘better’ - if it ain’t in the VA formulary, I’m not prescribing it.
This is actually really thoughtful. I’m from India, and we see a lot of hypertensive retinopathy here - often in younger people with poor access to follow-up. The idea of steady BP control over aggressive drops makes total sense. I’ve seen cases where patients got worse after being told to ‘lower it fast.’ The point about nocturnal dips? Spot on. We need more docs thinking like this - not just prescribing, but protecting. Thanks for writing this.
One is reminded of the ancient Indian medical tradition of ‘Rasa Shastra,’ wherein the balance of physiological forces - in this case, the interplay of systemic pressure and ocular perfusion - is understood not as a binary of high versus low, but as a dynamic equilibrium. The renin-angiotensin system, though recently identified in ocular tissues, has long been metaphorically aligned with the concept of ‘Apana Vayu’ - the downward-moving energy governing excretion and circulation. To suppress it indiscriminately is to disrupt the natural rhythm. Azilsartan, with its sustained action, may thus be viewed not as a mere antihypertensive, but as a modulator of ocular homeostasis - a subtle tuning of the body’s own regulatory orchestra. The absence of RCTs does not negate the wisdom of physiological alignment.
OMG I JUST READ THIS AND I’M CRYING 😭 I HAVE GLAUCOMA AND MY DOC JUST GAVE ME AZILSARTAN LAST WEEK AND MY VISION IS SO MUCH BETTER!!! I THINK IT’S WORKING!!! 🙏💖 #AzilsartanSavesEyes #NoMoreDrops #ScienceIsMagic
wait so azilsartan is like the new losartan? i thought losartan was the one for kidneys? did they make this to replace it or what? i read the part about 40-80mg but is it like twice a day or just once? my doc said once but i’m confused. also why no one talks about the side effects? i heard it makes you dizzy? ty for the info tho 😊
This is one of the most thoughtful, balanced, and clinically grounded posts I’ve read in months. You didn’t overstate, you didn’t oversimplify, and you acknowledged the gaps - which is rare. The emphasis on ABPM, nocturnal hypotension, and OCT tracking? That’s the future of personalized hypertension care. I’m a primary care doc in rural Maine, and I’m sharing this with my team. Thank you for taking the time to write this with such integrity. We need more of this.
Let me guess - this is all funded by Takeda. Azilsartan’s patent expires in 2028. They’re trying to create a new indication to extend exclusivity. The ‘ocular RAS’ stuff? Totally fabricated. They paid 3 neurologists to write papers linking angiotensin to ganglion cells. The ‘24-hour BP control’ advantage? Only measurable in their own trials. And now they’re pushing this on Reddit so doctors think it’s ‘evidence-based.’ I’ve seen the emails. They’re targeting neurologists and ophthalmologists. Don’t fall for it. This isn’t science - it’s corporate theater.